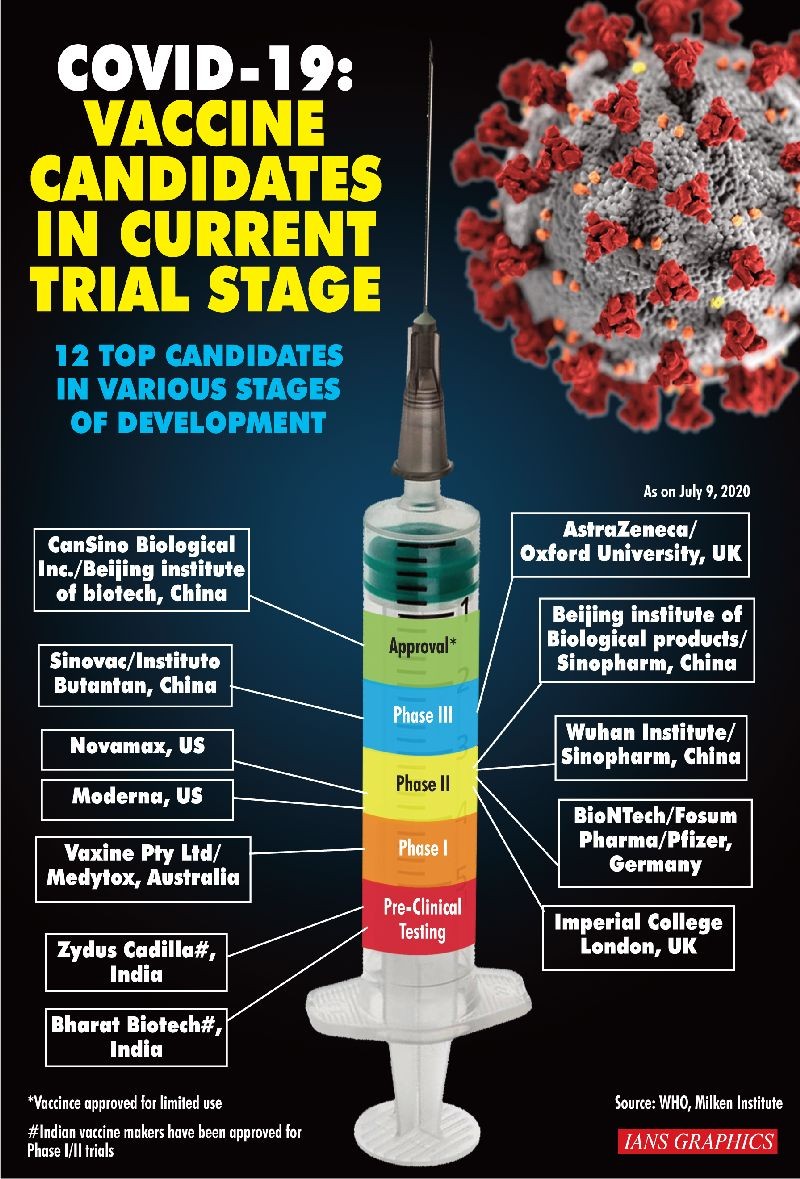
Beijing, July 21 (IANS) A phase 2 trial of a Covid-19 vaccine candidate conducted in China has found that the vaccine is safe and induces an immune response, according to a new study published in the journal The Lancet.
This comes barely 24 hours after on Monday The Lancet only published that a Covid-19 vaccine developed by scientists at the Oxford University produces strong immune responses in both parts of the immune system, showed results of Phase I/II trial.
The results from the Chinese trial provide data from a wider group of participants than the phase 1 trial, which was published in May. Phase 1 trial involved 108 healthy adults and it demonstrated promising results.
"The phase 2 trial adds further evidence on safety and immunogenicity in a large population than the phase 1 trial. This is an important step in evaluating this early-stage experimental vaccine and phase 3 trials are now underway," said study researcher Feng-Cai Zhu, Jiangsu Provincial Center for Disease Control and Prevention in China.
The Ad5 vectored vaccine in this trial uses a weakened human common cold virus (adenovirus, which infects human cells readily but is incapable of causing disease) to deliver genetic material that codes for the SARS-CoV-2 spike protein to the cells.
These cells then produce the spike protein and travel to the lymph nodes where the immune system creates antibodies that will recognise that spike protein and fight off the coronavirus.
According to the researchers, 508 participants took part in the trial of the new vaccine. Of these, 253 received a high dose of the vaccine, 129 received a low dose and 126 received placebo.
Approximately two-thirds of participants were aged 18-44 years, with a quarter aged 45-54 years, and 13 percent aged 55 years or older
Participants were monitored for immediate adverse reactions for 30 minutes after injection and were followed for any injection-site or systemic adverse reactions within 14- and 28-days post-vaccination.
The trial found that 95 per cent of participants in the high dose group and 91 per cent of the recipients in the low dose group showed either T cell or antibody immune responses at day 28 post-vaccination.
The vaccine-induced a neutralising antibody response in 59 per cent and 47 per cent of participants, and binding antibody response in 96 per cent of participants, in the high and low dose groups, respectively, by day 28.
The participants in the placebo group showed no antibody increase from baseline.
"Since elderly individuals face a high risk of serious illness and even death associated with COVID-19 infection, they are an important target population for a COVID-19 vaccine," said Wei Chen from the Beijing Institute of Biotechnology.
"It is possible that an additional dose may be needed in order to induce a stronger immune response in the elderly population, but further research is underway to evaluate this," Chen added.
The authors noted that the trial was conducted in Wuhan, China, and the baseline immunity is representative of Chinese adults at that time, but other countries may have different rates of immunity which should be considered.






