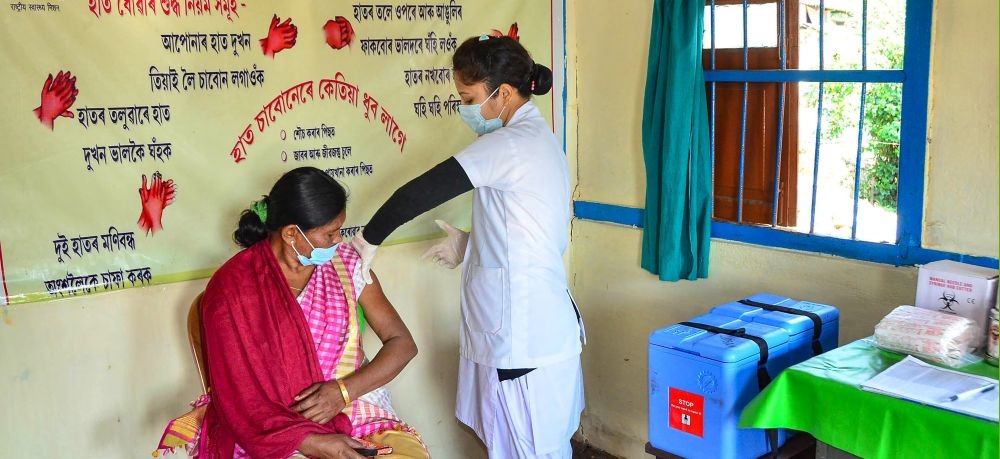
Sonitpur: A medic demonstrates administration of COVAXIN, an Indian government-backed experimental COVID-19 vaccine, to a health worker during its trials, at the Urban Primary Health Centre at Tezpur in Sonitpur district, Assam, Tuesday, Dec. 29, 2020. Dry run has been conducted successfully in four states of Andhra Pradesh, Assam, Gujarat and Punjab. (PTI Photo)
New Delhi, December 29 (IANS): The two-day dry run for the Covid-19 vaccination in Assam, Andhra Pradesh, Punjab and Gujarat has been successfully conducted, the Union Ministry of Health and Family Welfare said on Tuesday.
The end-to-end dry run was conducted in Krishna district in Andhra Pradesh, Rajkot and Gandhinagar districts in Gujarat, Ludhiana and Shaheed Bhagat Singh Nagar in Punjab and Sonitpur and Nalbari districts in Assam on December 28 and 29.
The District Collector with engagement of district and block task force were made responsible for conducting the dry run and were expected to provide insights on any gaps or bottle-necks during the actual conduct of vaccination.
Specific teams were formed for various tasks by the district administration and activities such as uploading of dummy beneficiary's data, session site creation, vaccine allocation, communication of vaccination details to vaccinators and beneficiaries and beneficiary mobilisation were carried out.
Field feedback on the first day of the run was reviewed today through video conferencing with state and district programme officers by Joint Secretary (Public Health). All states expressed satisfaction in terms of the operational approach and use of IT platform to ensure transparency and effective monitoring of vaccination processes expected to cover a large number of people across the country.
Additional suggestions on the IT platform were also noted for further enhancement of Co-WIN platform. Detailed insights and feedback so obtained will help enrich the operational guidelines and IT platform and will strengthen the Covid-19 vaccination roll out plan, the ministry said in statement.
"Backed with the experience of rolling out the Universal Immunization Programme (UIP) and conducting nationwide multiple wide-range injectable vaccination campaigns such as measles-rubella (MR) and adult Japanese Encephalitis (JE) campaign, required steps are being undertaken to vaccinate priority population groups such as health care workers, frontline workers and people above 50 for Covid-19," the statement read.
The dry run exercise was aimed at end-to-end testing of the Covid-19 vaccination process and included planning and preparations as per the Operational Guidelines, creation of facilities and users on Co-WIN application, session site creation and mapping of sites, Health Care Workers (HCW) data upload, receipt of vaccines and vaccine allocation by the district, session planning, deployment of vaccination team, logistics mobilisation at session site, mock drill of conducting vaccination and reporting and review meetings at block, districts and state level.
The objective of the dry run was also to undertake and confirm field implementation of IT platform Co-WIN and guide the way forward prior to actual implementation.
After a year which saw an unprecedented global health crisis, many are anticipating the coronavirus vaccine as India prepares for the inoculation campaign, which is likely to commence in January 2021.
India currently has eight Covid-19 vaccine candidates, including three indigenous vaccines, under different stages of clinical trials which could be ready for authorisation in near future. Serum Institute-Oxford's Covishield, Bharat Biotech's Covaxin and Pfizer vaccine are in the fray for emergency use authorisation.
The central government plans to vaccinate nearly 30 crore people in the first phase of drive. It will be offered to one crore healthcare workers, along with 2 crore frontline and essential workers and 27 crore elderly, mostly above the age of 50 years with co-morbidities.