New Delhi, October 10 (PTI): The cumulative COVID-19 vaccine doses administered in the country surpassed the 95-crore mark on Sunday, the Union Health Ministry said.
More than 44 lakh vaccine doses have been
Support The Morung Express. Your Contributions Matter Click Here
Covid-19 vaccine
- New Delhi, October 9 (IANS): Health Minister Mansukh Mandaviya on Saturday asked the states to focus on working towards achieving the nation's 100-crore Covid-19 vaccine doses goal. India has so far adminis
- London, September 14 (PTI): Schoolchildren aged between 12 and 15 years will be offered a first dose of the Pfizer/BioNTech COVID-19 vaccine starting next week, the UK government has announced after the country
- New Delhi, September 2 (PTI): Around 80 per cent teaching and non-teaching staff employed in schools across the country have received at least the first dose of COVID-19 vaccine, Education Ministry officials sa
- London, September 2 (PTI): People infected with the SARS-CoV-2 virus after receiving one or two COVID-19 vaccine doses have significantly lower chance of severe disease or hospitalisation than unvaccinated
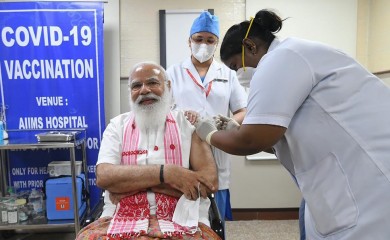
- New Delhi, March 1 (IANS) Prime Minister Narendra Modi received the first dose of Covid vaccine here at All India Institute of Medical Sciences (AIIMS) on Monday, leading the country in the third phase of the i
- Kohima, February 24 (MExN): COVID-19 vaccination consultation workshop with civil societies and church organizations was held at Conference Hall, Medical Directorate, Kohima on February 24. A DIPR report sta
- Singapore, February 20 (IANS): Covid-19 vaccinations for people aged 60 to 69 will start around the end of March, with the rest of the population to follow suit in April, said Health Minister Gan Kim Yong on Fr
- London, February 7 (PTI): The COVID-19 vaccine developed by Oxford University and produced by AstraZeneca has shown efficacy against the UK variant of the coronavirus, according to an ongoing study by researche
- New Delhi, January 28 (IANS): While India has already given Covid-19 vaccine doses to over 20 lakh people as the first phase of the drive continues, a new study has said that the vaccination programme against t
- New York, January 25 (IANS): US-based drugmaker Moderna on Monday said its Covid-19 vaccine retains neutralising activity against emerging variants first identified in the UK and South Africa. Vaccination wi
- San Francisco, January 25 (IANS): Microsoft founder and philanthropist Bill Gates, who has been a target of conspiracy theorists for his push for vaccinations, has received the first dose of a Covid-19 vaccine.
- Newmai News Network Imphal | January 18 There has been no report of any side effect regarding the COVID-19 vaccine since the start of vaccination drive in Manipur on January 16, Chief Minister N Biren
- Aurangabad, January 16 (PTI): Prime Minister Narendra Modi should first get himself vaccinated for COVID-19 to clear doubts in the minds of people, Vanchit Bahujan Aghadi (VBA) leader Prakash Ambedkar said on S
- Morung Express News Dimapur | January 16 Blood Bank incharge of District Hospital Dimapur, Dr Temsu, who received the first dose of COVID-19 vaccine in Dimapur described his experience as “painless.&r
- Kohima, January 16 (MExN): Under urban areas in Kohima district, a total of 21 sites, 16 at government health facilities and 5 at private health facilities have been set up for vaccination sessions. Accordin
- DIMAPUR, JANUARY 14 (MExN): The Chief Medical Officer of Dimapur, Dr Mereninla Senlem today stated that Nagaland State has very good storage facilities maintaining the required 2 to 8 degree Celsius storage tem
- Morung Express News Dimapur | January 14 In a positive development, Nagaland today received the first consignment of the COVID-19 vaccines, two days ahead of the scheduled roll-out on January 16.
- New Delhi, January 12 (PTI): There will be a gap of 28 days between two doses of COVID-19 vaccine and its effectiveness will begin 14 days after the second dose, the Health Ministry said on Tuesday. Addressi
- New Delhi, January 7 (PTI): The Union health ministry has informed states and union territories that they are likely to receive the first supply of COVID-19 vaccine shortly and asked them to remain prepared to










.jpg)



