Morung Express News
Dimapur | May 8
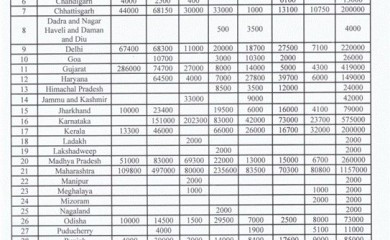
The combined allocation of antiviral drug Remdesivir to seven North East States excluding Assam was less than the single share for all other States in India, revealed the
Support The Morung Express. Your Contributions Matter Click Here
Remdesivir
- ZURICH/GENEVA, October 16 (Reuters): A World Health Organization (WHO) trial that concluded Gilead Sciences Inc. remdesivir did not significantly help COVID-19 patients is reliable, a scientist who evaluated it
- AMSTERDAM/WARSAW/BRUSSELS, October 6 (Reuters): European countries are facing shortages of COVID-19 drug remdesivir because limited supplies are running out, officials said, with cases surging and the Unit
- New Delhi, August 13 (PTI): Drug firm Zydus Cadila on Thursday said it has launched Remdesivir under the brand name Remdac, used to treat patients suffering from severe symptoms of COVID-19, in the Indian marke
- Reuters Doctors in Europe will soon be able to treat COVID-19 patients with Gilead's antiviral drug, remdesivir, after the healthcare regulator's endorsement put it on track to become the f
- Reuters Gilead Sciences Inc's antiviral drug, remdesivir, prevented lung disease in macaque monkeys infected with the new coronavirus, a study published in medical journal Nature showed on Tuesday. &n
- BENGALURU, June 2 (Reuters): India's government said on Tuesday it has approved Gilead Sciences Inc's antiviral drug remdesivir for emergency use in treating COVID-19 patients. Remdesi